The coadministration of anticoagulation and thrombolytics
Anticoagulation should be started immediately when administering thrombolytic therapy to a patient with a STEMI, we give both together. The same applies to PE with hemodynamic instability.
This is different from using thrombolytics in ischemic stroke! In ischemic stroke, the American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend delaying the initiation of anticoagulation for 24 hours after thrombolytic therapy with alteplase to minimize the risk of hemorrhagic transformation.
Holding anticoagulation before procedures
Right-sided thoracentesis, paracentesis, central line placement including picc lines, diagnostic endoscopy with or without biopsy, dental procedures, minor dermatological procedures, ophthalmological procedures, and pacemaker and ICD placement are considered minor procedures and considered low risk for bleeding.
The American College of Chest Physicians guidelines support the continuation of anticoagulants for minor procedures due to their low bleeding risk. The same applies to antiplatelets.
In real-world settings, unless the procedure is emergent, I highly recommend checking with the performing physician to see if we should hold oral anticoagulants to avoid unnecessary delays! Unfortunately, not everyone goes with these guidelines for different reasons!
Suppose the performing provider wants to hold oral anticoagulants. In that case, we hold DOAC for 48 and hold warfarin until the INR falls to a level that is deemed safe by the performing physician which is typically ≤ 1.5.
Anticoagulation bridging
Bridging with UFH or LMWH is warranted in patients who are taking warfarin and have a high risk for thromboembolic disease such as mechanical heart valves, AF patients with recent stroke or TIA, recent DVT or PE, and patients with known hypercoagulable status.
Bridging is initiated when the INR falls below the therapeutic range typically less than 2.
Bridging is not warranted for DOAC given their short half-lives and rapid onset and offset unless the therapy interruption is going beyond 48 hours.
LMWH is preferred over UFH in bridging given its more predictable anticoagulation effect and should be stopped 24 hours before the procedure, If UFH is used instead, it should be stopped 4-6 hours before the procedure.
Resuming oral anticoagulants
Oral anticoagulants should be resumed after the procedure and I always ask the performing physicians if it is safe to resume. DOAC can be simply resumed, and the same applies to warfarin if bridging isn’t indicated. If bridging was indicated, it should continue until the INR is therapeutic. Here’s the transitioning strategy we follow:
- From UFH: Start the DOAC at the time of heparin drip discontinuation.
- From LMWH: Start the DOAC when the next LMWH dose is due.
- No loading dose is needed when resuming a DOAC—simply restart the home dose.
Heparin-induced thrombocytopenia
HIT is a serious condition with significant morbidity and mortality if untreated and one should not wait for thrоmbоsis to develop before suspecting НΙТ because thrοmbοϲуtοpeոiа often precedes that.
Calculating the 4T score is the first step when suspecting HIT. this score is a clinical tool used to estimate the probability of heparin-induced thrombocytopenia (HIT). It evaluates four parameters related to HIT and stratifies patients into low, intermediate, or high probability categories. This scoring system helps guide further diagnostic testing and management. You don’t need to memorize it, just use one of the free online calculators.
If the 4 Ts score is a low probability, we generally do not pursue HΙΤ antibody testing or presumptive treatment for НІΤ, we do not discontinue hepariո or start a non-heparin anticoagulant
If the 4 Ts score is an intermediate or high probability, we make a presumptive diagnosis of ΗІТ pending results of НΙT antibody testing.
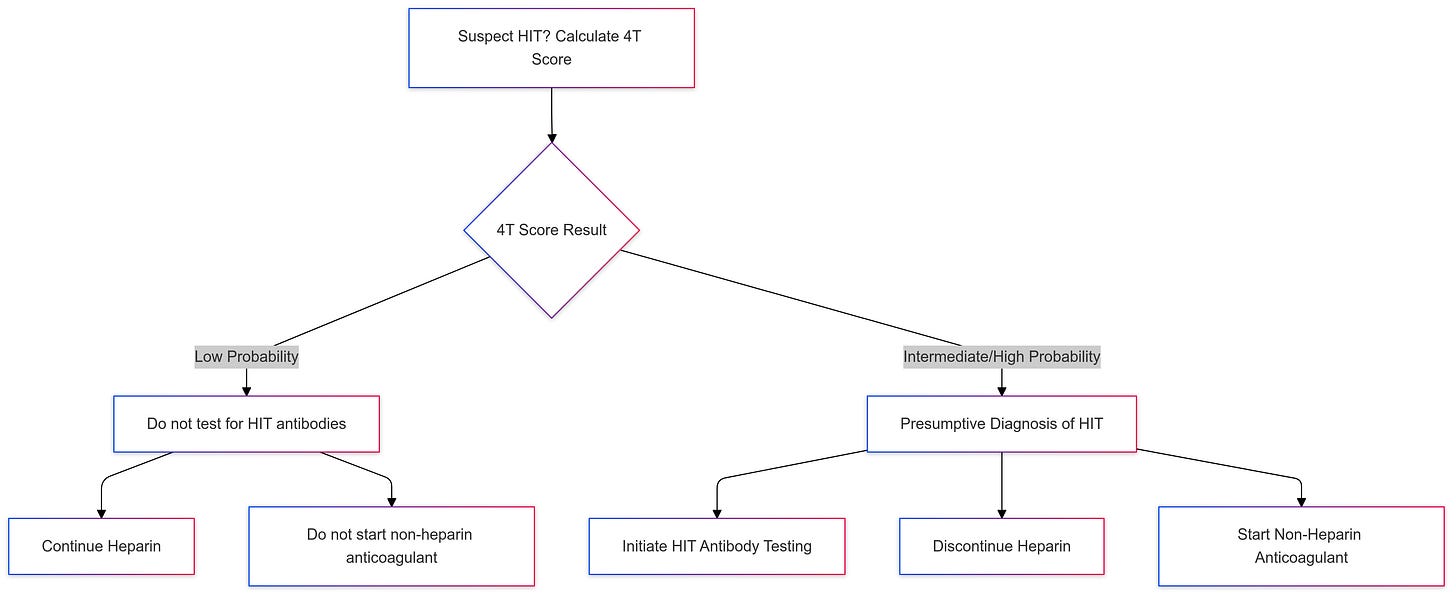
Нерariո cessation alone is not sufficient since patients with НΙТ remain at risk for subsequent thrоmbοsis. So If you decide to test for HIT, you must act pending test results.
Testing for HIT starts with screening antigen assay tests like ELISA, which detect antibodies against platelet factor 4 (PF4)/heparin complexes. These assays are fast and highly sensitive but have moderate specificity, leading to potential overdiagnosis.
A negative result will rule out HIT, but if positive we send a confirmatory functional assay unless the clinical suspicion is high then there is no need.
Non-heparin anticoagulants include argatroban, bivalirudin, fondaparinux, or a direct oral anticoagulant (DOAC). Remember the use of Fodaparinux and DOAC in HIT is considered off-label.
Argartroban and bivalirudin have short durations of action and are good choices for critically ill patients. Argatroban is safe in renal insufficiency, while bivalirudin is good in liver dysfunction.
Fondaparinux is a good and easier-to-use option but is contraindicated in patients weighing less than 50 kg and those with a creatinine clearance below 30 ml/min. It’s also best avoided when the platelet count is below 50,000/µL.
DOAC agents are best used in stable patients, they are equally effective. Apixaban is the DOAC of choice in renal insufficiency and dialysis patients. A loading dose of DOAC is required similar to treating venous thromboembolic disease.
In cases HIT is confirmed, the duration of anticoagulation therapy should be at least 4 weeks for isolated HIT and 3 months for HIT with thrombosis.
In the case of using Argatroban and Bivalirudin, have your pharmacist help with their protocol and monitoring and transition to oral anticoagulants once the patient is stable preferably DOAC, if you decide to transition into warfarin we must overlap until the INR is therapeutic for at least 24 hours.
Anticoagulation reversal
For warfarin-associated life-threatening bleeding, 4-factor Prothrombin Complex Concentrate (4-factor PCC) which contains factors II, VII, IX, and X is the first-line reversal solution, if not available, we can use the combination of 3-factor PCC and factor 7 as factor-3 PCC lacks factor 7. FFP is used when PCC solutions are not available.
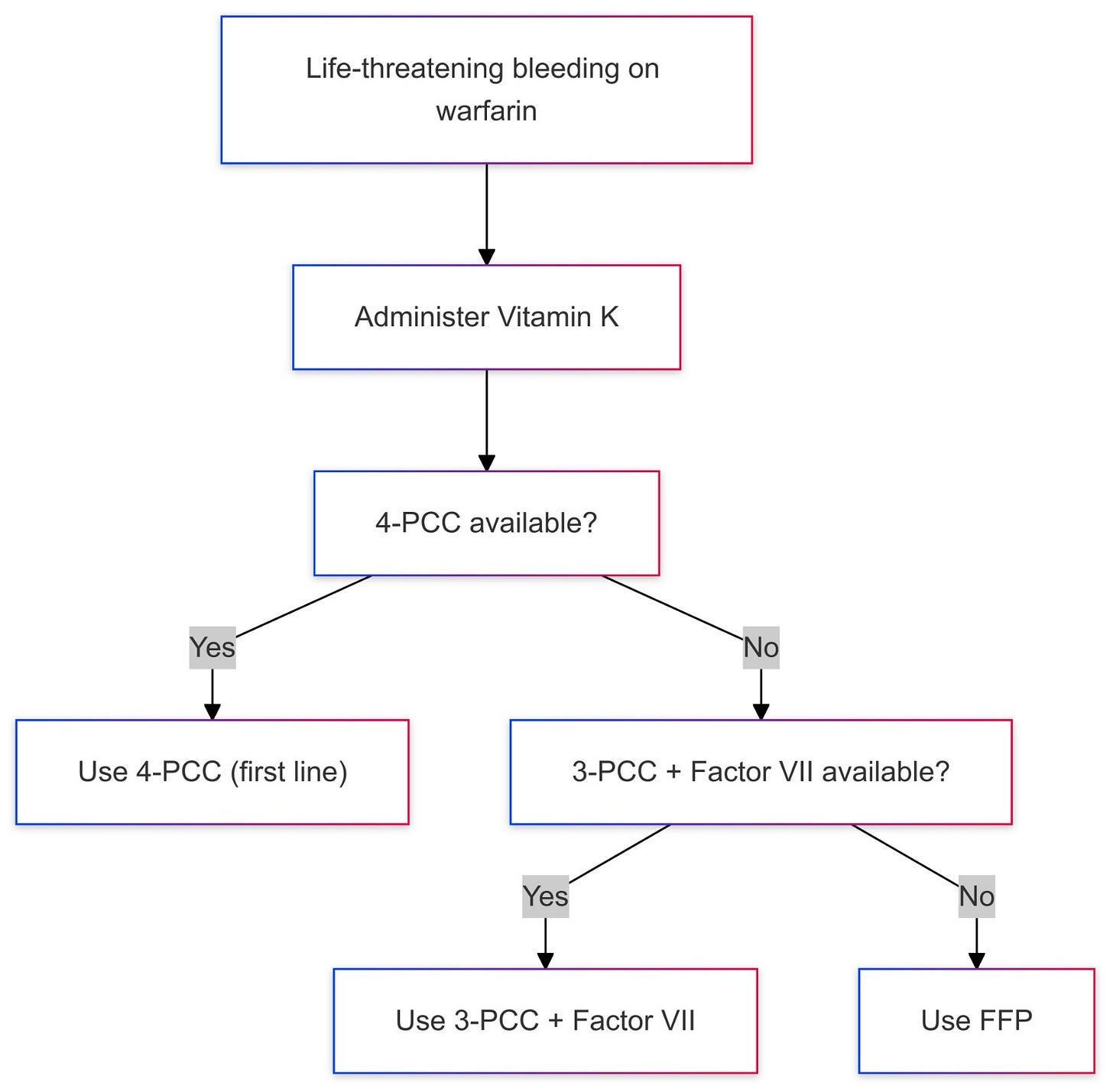
Warfarin is still the drug of choice in mechanical valves, A. fib with moderate to severe mitral stenosis, and antiphospholipid syndrome.
In most cases, the goal is to bring INR to ≤ 1.5. Check the INR level 15-30 minutes after completion of the infusion and repeat the infusion until INR is ≤ 1.5, repeat the infusion if INR is still elevated.
In general, it is reasonable to consider restarting anticoagulation therapy 7 to 14 days after an episode of major bleeding.
Final approval should always be secured from the subspecialists overseeing the bleeding management.
For DOAC reversal, we follow the following strategy:
• Dabigatran: Idarucizumab is the first line, or PCC/aPCC, if Idarucizumab is unavailable. If PCC is not available then HD may be considered to remove the drug as the next step.
• Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban): Andexanet alfa (preferred), or PCC/aPCC if andexanet alfa is unavailable.
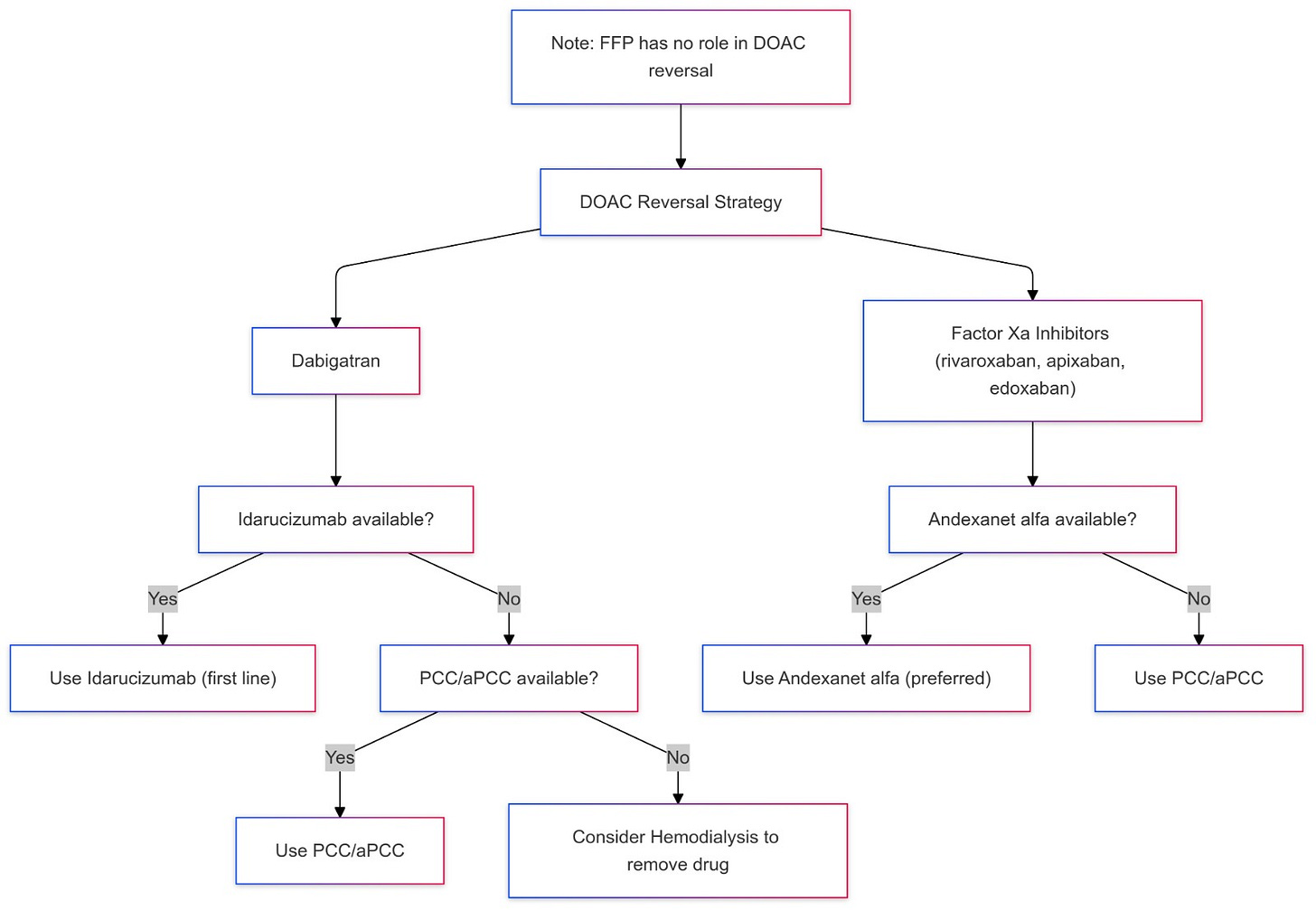
FFP has no role in DOAC anticoagulation reversal.
Protamine sulfate is the primary agent to reverse the anticoagulation effect of UFH and LMWH in life-threatening bleeding.
Anticoagulation and bleeding
In cases of significant bleeding, the immediate priority is to discontinue all anticoagulation. The next step is to reverse the coagulopathy when clinically appropriate.
In cases of minor bleeding, such as gum bleeding, epistaxis, bloody stools from inflammatory bowel disease (IBD), microscopic hematuria, or hemoptysis caused by a lung abscess, anticoagulation can often be continued with caution. However, addressing the underlying cause of the bleeding is essential. Importantly, if hemoptysis is due to pulmonary embolism (PE), anticoagulation should not be withheld, as treating the PE is the priority.
n thrombocytopenia is drug-induced, plt transfusion is indicated in active bleeding to keep the plt count above 50k.
Anticoagulation can be resumed 7-14 days after a major bleeding episode.
For anticoagulation in critically ill patients and those at high rebleeding risk, UFH infusion is preferred given its short duration of action. LMWH or DOAC can be considered when the patient becomes more stable and low bleeding risk.
A platelet count of ≥ 50k is considered safe to start anticoagulation, a lower threshold of ≥ 30k can be considered if the benefit outweighs the risk of starting anticoagulation.
The IVC filter debate
The use of inferior vena cava (IVC) filters is a topic of considerable debate in the medical community. IVC filters are indicated primarily for patients with venous thromboembolism (VTE) who have an absolute contraindication to anticoagulation or in those who have recurrent VTE despite adequate anticoagulation. This recommendation is supported by the American Society of Hematology (ASH) and the Society of Interventional Radiology, among others.
However, there are significant concerns regarding the routine use of IVC filters due to the associated risks and limited evidence of long-term benefits. Studies have shown that while IVC filters can reduce the incidence of pulmonary embolism (PE) in the short term, they are associated with an increased risk of deep vein thrombosis (DVT) and other complications such as filter migration, perforation, and thrombosis.
The American Society of Hematology guidelines conditionally recommend against the use of IVC filters in patients who can be safely treated with anticoagulant therapy, citing low certainty in the evidence of their effects. Similarly, the Society of Interventional Radiology advises that IVC filters should be used selectively and recommends the use of retrievable filters, with removal once the patient can be safely anticoagulated.
In summary, while IVC filters have a role in specific clinical scenarios, their use should be carefully considered, weighing the potential benefits against the risks. The current consensus advises against their routine use in favor of anticoagulation whenever possible.






Transfusion Medicine Made Simple: Essential Guide for Clinicians
Eight EKG patterns in acute MI we can’t afford to miss!
The top three antiemetics I rely on!
The use of 3% NS in hyponatremia, when and how.
The inpatient treatment of hypercalcemia
Hyperkalemia-induced EKG changes
The Proper Way to Replace Magnesium
Non-insulin diabetic medications